Abstract
Background: Limited-stage follicular lymphoma (FL), defined as stage I or II disease, is generally treated with radiation therapy (RT) to involved nodes with excellent overall response rates. However, nearly half of all limited-stage FL patients will ultimately experience a relapse. Most prior research on genetic prognostication in FL has focused on patients with advanced disease undergoing systemic therapy, however, there is evidence to suggest that there are genetic differences in limited versus advanced-stage FL, for example, in the frequency of KMT2D and BCL2 mutations as well as gene expression profiles.
Objective: We hypothesized that genetic characterization of pre-treatment biopsies can identify those limited-stage FL patients that are at high risk of relapse following radiation therapy.
Methods: Pre-treatment formalin-fixed and paraffin-embedded tissue block samples were obtained from adult patients with limited-stage FL, grade 1-3A, treated with curative-intent RT diagnosed between 1995-2018, from 6 international sites in Canada, Europe and Australia. Hybridization-based targeted sequencing of a 71 gene panel was performed, which included genes that are known to be recurrently mutated in FL. Samples with >50X mean on target coverage were retained in the analysis. Variant calling was performed using Mutect2. Germline polymorphisms were filtered out by cross-referencing with dbSNP and gnomAD databases. Only coding, non-silent mutations detected at a variant allele frequency greater than 0.1 were considered. Patient demographics and clinical data were examined retrospectively. Genes mutated at a proportion of 10% or more of the cohort were tested for association with time to progression (TTP) and overall survival (OS).
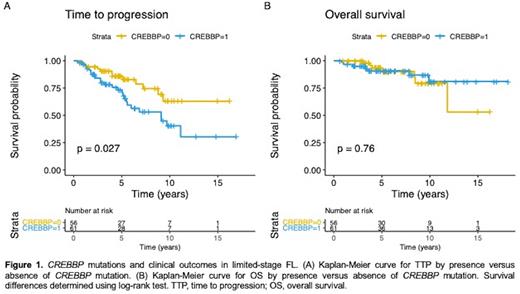
Results: One hundred and seventeen pre-treatment samples from distinct patients were accrued. The median age was 58 years (range: 26-86), and 68 (58%) were female. There were 99 (85%) patients with stage I disease, and 18 (15%) with stage II. Grade was 1 or 2 in 86% of patients. Mean maximum tumour diameter was 3.33cm (range: 0.5-8cm). Of 110 patients with FLIPI scores available, 100 patients had a low FLIPI score (91%), 9 (8%) had an intermediate score, 1 (1%) patient had a high score. Patients were staged with cross-sectional imaging, including a PET scan in 73 (63%) of cases, and bone marrow assessment was completed in 105 (90%) of patients. The most commonly mutated gene in the cohort was CREBBP (n=61; 52.1%), followed by KMT2D (n=45; 38.5%), TNFRSF14 (n=40; 34.2%), STAT6 (n=25; 21.4%), and BCL2 (n=20; 17.1%). BCL2 mutations were more common in patients with stage II (n=8; 44%) compared to stage I disease (n=12; 12%) (p-value=0.003; after FDR adjustment, p-value=0.056). The median number of mutated genes per patient was 4 (range: 0-12, mean 4.0). The median follow-up of living patients was 5.65 years (range: 0.63-18.1 years) and there were 37 progression events in our cohort. CREBBP mutations were associated with shorter TTP (log-rank p-value 0.027), with 5-year TTP estimates of 73.3% vs. 86.0% (Figure 1A). CREBBP mutations were not significantly associated with age, stage, tumor bulk or PET staging. Using Cox univariable analysis for TTP, the hazard ratio (HR) for CREBBP mutations was 2.13 (95% confidence interval (CI) 1.07-4.25; p-value=0.031) and in multivariable analysis adjusting for age and sex the HR was 2.05 (95%CI 1.03-4.10; p-value= 0.041). However, statistical significance was not met after adjusting for the false discovery rate (FDR; p-value 0.621). CREBBP mutations were not significantly associated with OS (log-rank p-value=0.76) (Figure 1B), nor transformation (log-rank p-value=0.9), but the numbers of survival (n=14) and transformation events (n=9) were small. No other genes from the panel were associated with patients' outcomes.
Conclusions: In conclusion, we found an association between CREBBP mutations and shorter time to progression in limited-stage FL treated with RT. To our knowledge, this represents a novel genetic prognostic finding in limited-stage FL. Further studies with larger sample sizes are required to confirm the association.
Disclosures
Baetz:Servier: Honoraria; Roche: Honoraria; Gilead: Honoraria; Astrazeneca: Honoraria; BeiGene: Honoraria. Johnson:Merck, AbbVie, Roche, Gilead: Consultancy. Crump:Kyte/Gilead: Honoraria; Novartis: Honoraria; Roche: Research Funding. Kukreti:Kwoya Kirin: Honoraria; EUSA Pharma: Honoraria. Kuruvilla:Antengene: Consultancy; Novartis: Honoraria; Janssen: Honoraria; Medison Ventures: Consultancy; Amgen: Honoraria; Abbvie: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria, Other: DSMB; Astra Zeneca: Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Inctye: Honoraria; Pfizer: Honoraria; Lymphoma Canada: Membership on an entity's Board of Directors or advisory committees. Kridel:Abbvie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal